How far we've come since Yuri Gagarin first went to space 64 years ago
Cosmonaut Yuri Gagarin was the first human to enter space.
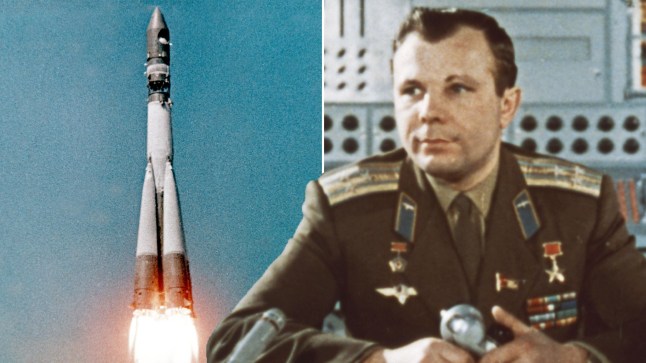
Cosmonaut Yuri Gagarin was the first human to enter space.

Barcelona Bridal Fashion Week presents its largest and most ambitious edition with to become a true powerhouse of creativity,business and innovation in the field of bridal,ceremony and red carpet fas

Kampala Declaration calls for scaling up Forward Faster initiative across Africa KAMPALA,Uganda,April 12,2025--To accelerate progress towards achieving the Sustainable Development Goals (SDGs) in Afr

We are advised by Visionary Holdings Inc. that journalists and other readers should disregard the news release,Visionary Seizes the Primary Resource in the New Energy Vehicle Sector and Goes All Out t

OSAKA,Japan,April 13,2025-- On April 13,2025,the Expo 2025 Osaka in Japan officially opened,along with the China Pavilion held its opening ceremony. Representatives from the political and business com

CHENGDU,China,April 12,2025-- SichuanKelun-Biotech Biopharmaceutical Co.,Ltd. (the "Company") today announced that results from a Phase 3 registrational clinical study evaluating the novel

The partnership and $100 million investment positions Aiper to scale operations and solidify its role as a leading player in smart pool cleaning technology by uniting complementary strengths to delive

WASHINGTON,April 14,2025-- The International Copper Association (ICA) proudly welcomes its newest member,The Weir Group (Weir)—a global leader in mining technology and innovation. This strategic part

FIRSTHABIT is launching a strategic recruitment drive targeting a double-digit increase in its workforce,aiming to secure top-tier talent to revolutionize the global AI education sector.

HANGZHOU,China,April 14,2025--In April 2025,Guozi Robotics and U.S.-based Staples celebrate the ninth year of their strategic partnership. Over the course of this collaboration,the two companies have